Silver Corrosion in Liquid-filled Transformers
Authors
Jelena LUKIC, Gordon WILSON, Ivanka ATANASOVA-HOEHLEIN, Edward CASSERLY, Dejan VUKOVIC, Pieter DE BIJL, Brendan DIGGIN, Hongzhi DING, Chadia EL KADI, Ronny FRITSCHE, Jelena JANKOVIC, Lance LEWAND, Diego LUMBRERAS, Dijana VRSALJKO, Marco MILONE, Volker NULL, Christophe PERRIER, Konrad RÄDLINGER, Mahdi RAHMBEKSCH, James REID, Adam SULEIMAN, Ed VAN SCHAIK, Veronika HARAMIJA, Mark LASHBROOK, Rainer FROTSCHER

Summary
CIGRE SC A2 has responded to reports in the industry of new cases of corrosive sulphur in transformers. A new Task Force has collected cases of silver corrosion in tap-changers resulting from sulphur compounds in synthetic ester and mineral oil. Investigations by TF members suggest that there is a need to improve the tests currently carried out for silver corrosion and corrosive sulphur. The TF proposes a new Working Group is needed to identify causes and assess risk for transformer owners.
Introduction, Objectives of this Technical Brochure
Historically, the phenomenon of copper corrosion in liquid-filled power transformers has garnered significant attention within the CIGRE community [1, 2], primarily due to the elevated risk of failure associated with the deposition of copper sulphide on paper insulation. Prompt intervention by CIGRE members facilitated the swift identification of the underlying causes, the development of risk assessment methodologies, and the evaluation of mitigation strategies, thereby bringing the issue under control. Conversely, incidents of silver corrosion, despite posing potentially greater risks in certain scenarios, received comparatively less focus due to their less frequent occurrence.
For decades, the challenge of silver corrosion detection in transformers has been acknowledged, with several standards, including DIN 51353 [3, 4] and ASTM D1275 [5, 6], establishing testing protocols to ascertain the corrosive potential of insulating oil. Moreover, the IEC 62697 series introduced quantitative tests to measure the concentrations of various sulphur compounds, enhancing understanding and detection capabilities [7, 8].
Traditionally, instances of silver corrosion were predominantly linked to the presence of reactive sulphur in mineral oil, emanating from inadequate refining processes, contamination, or flawed oil reclamation practices [9]. Recent incidents with synthetic esters have underscored a gap in the collective understanding of silver corrosion mechanisms and have questioned the adequacy of existing measures to prevent such failures, thereby indicating a need for further investigation and potential revision of current practices.
Scope/Methodology
This is a report from the activity of CIGRE Study Committee A2 (Transformers and Reactors) Task Force 2 "Silver Corrosion". The scope of TF A2.02, which was established in April 2024, was to:
- Collect and document recent cases of silver corrosion
- Investigate the root causes and the origin of corrosive agents
- Propose appropriate prevention and mitigation measures
The scope of this article is therefore to share with the wider community some details of the cases that were contributed by the invited members. The second and third tasks have been explored but the TF has not been able to reach firm conclusions in the time available. The presence of elemental “crown” sulphur (S8) appears to be the cause of silver corrosion in cases where synthetic ester is involved. S8 has been identified as a cause of silver corrosion in transformers previously [2, 9, 10, 11] with the source being identified as improper oil regeneration using reactivatable bauxite clay (primarily aluminium oxide). During the reactivation step the remaining oil on the column may reach very high temperatures (up to 900°C), the oil is thereby “cracked” (breaking hydrocarbons to smaller molecules) and S8 may be formed from sulphur-containing molecules. If this is not drained and instead reaches the transformer it may not be completely removed during further regeneration activity.
New cases of silver corrosion in mineral oil-filled transformers have been reported but regeneration has not been involved and neither, it seems, is S8. Rather, the previously identified [1, 12, 13] cause of copper sulphide formation in transformers, dibenzyl disulphide (DBDS), may be playing a part. The reaction between copper and sulphur may be mitigated by the addition of a passivator, but the same passivators are not effective with silver. It has also been suggested that sulphur may be coming from the rubber [2, 14, 15, 16], since sulphur is used in the curing process of nitrile butadiene rubber (NBR) that can be used in the construction of some transformers and is a quicker curing process than using peroxides. At this stage of the investigation, other materials cannot be excluded.
As an indication of compatibility between insulating liquids and other materials, this TF finds that silver corrosion tests would benefit from greater sensitivity. In addition, measurements of sulphur content, primarily reactive disulphides, DBDS and S8, at low levels can support understanding of adverse results where they arise.
The TF is recommending that the work continues in a CIGRE Joint Working Group (JWG) between SC A2 and SC D1 (Materials and Emerging Test Techniques). The group has conducted much laboratory study on detecting corrosive sulphur and quantifying S8 at low concentrations, which is detailed below. The JWG will be able to settle on recommended conditions for standard methodologies for silver corrosion detection and quantitative sulphur tests and it is therefore necessary that SC D1 is also involved. ASTM is well represented in the TF and will be reviewing the relevant published standard.
The proposed JWG would not only continue the testing work but also support transformer owners with risk assessment guidance for addressing potential problems with transformers already in service. Various risk assessment methodologies may be undertaken and there are a wide range of parameters to consider. CIGRE would be able to add significant value in providing users with information to navigate the options and various technical factors to understand any actions they need to take.
Case Studies
Members of the TF reported instances where silver corrosion was observed in their own transformers or that were shared with the permission of their owners. Note that operational issues directly related to silver corrosion were not observed in all cases. The phenomenon is relatively new and still under investigation in many cases, so it is not possible to share full details of these cases. Even presented in brief, the details below illustrate that there is an issue in need of further study.
Cases in mineral oil
In the first case, a TF member observed that transformers purchased between 1998-2007 supplied with uninhibited mineral oil had corrosive sulphur from DBDS. The majority of the transformers were passivated to mitigate the copper sulphide issue. On inspection, a transformer with abnormal dissolved gas analysis (DGA) results showed silver corrosion within the selector switch contacts (in tank selector switch design) (Fig. 1) and electrical treeing on the fibreglass support structure. Inspections of other transformers were then undertaken to determine whether this was a common issue. Varying levels of silver corrosion were found when these were carried out.
The DBDS level varied between 20 and 200 mg/kg in these transformers, and they had been passivated using a commercially available concentrate of 20% toluyl triazole (TTA) in mineral oil. The average loading on the transformers is estimated to be around 30%. Thus far, ASTM D1275 silver strip testing has not been carried out although copper strip testing has been carried out.
Where silver sulphide has been identified corrective actions have been implemented successfully: conducted an internal risk assessment, prioritise the mitigation work; on-site oil processing and manual cleaning of the silver contacts.
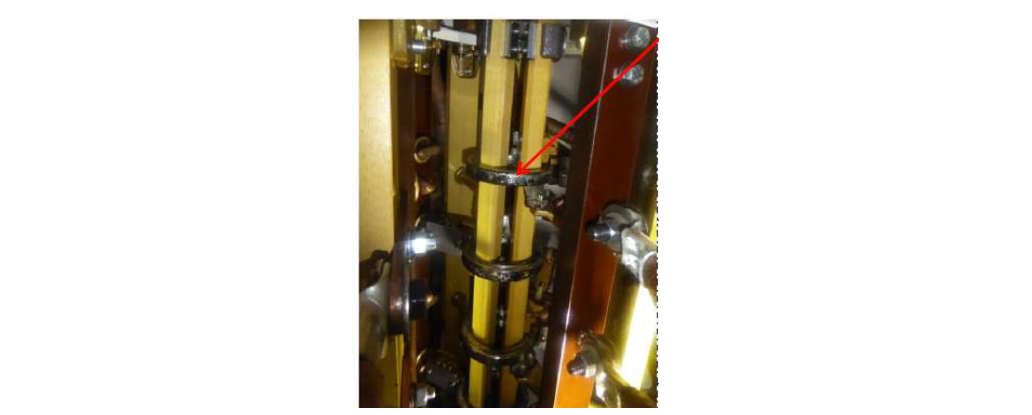
Figure 1 - Blackened silver surfaces on in-tank selector
Another new case of corrosion in mineral oil was reported involving a 40-year-old 300/23 kV 735 MVA generator step-up (GSU) transformer originally filled with mineral oil containing excessive amounts of potentially corrosive sulphur (i.e. DBDS) that had also been passivated. Approximately 18 months after passivation, DGA results indicated a severe overheating fault in the red phase selector and not in the main tank and other two selectors. Internal inspection discovered significant copper and silver whiskers in the faulty changeover contact, as shown in Fig. 2. The passivator content was found to still be at 72 mg/kg.

Figure 2 - Copper and silver whiskers in the faulty changeover contact of a GSU selector
In another case reported to the TF, a mineral oil-filled transformer manufactured in 2016 tripped in 2024. On inspection a flashover between contacts of the on-load tap changer (OLTC) were noted. The silvered contacts were noted to be significantly blackened in some areas and this was identified as silver sulphide. The suspected cause of failure is reduced dielectric strength between contacts caused by silver sulphide flakes bridging the gap.
A sister unit of the failed transformer on the site was inspected and all silvered contacts were found to be heavily blackened (Fig. 3). Twenty-four sister units in total require inspection, so far five have been inspected and three were found blackened while two were not. Those found to be clear of corrosion were manufactured more recently (2017).
It was suggested to the utility that the source of the sulphur could be from the curing process of rubber and that more recent tap-changers are less likely to suffer from the problem as rubber cured using an alternative method will have been used [16].

Figure 3 - Blackened silver contacts from transformer removed for inspection following failure of sister transformer
Another TF member reported on a fleet of approximately 20 transmission transformers manufactured between 2010 and 2017 which required an internal inspection due to a type-fault on the high voltage (HV) bushing corona shields. The transformers were filled with an uninhibited mineral oil. The transformers are free breathing with silica gel breathers installed on the conservator tank and a separate breather on the diverter switch of the OLTC.
During some of these inspections, blackened surfaces were noticed on the silver-plated OLTC selector switch contacts in the main tank compartment (Fig. 4). These transformers have a resistor-type diverter switch OLTC which has its own oil compartment.

Figure 4 - Black deposits on selector switch OLTC contacts
No gassing activity was noticed in any of the transformers affected by these blackened areas on the selector switch contacts. The oils tested negative for silver corrosion according to ASTM D1275 but failed a modified silver corrosion test performed by the laboratory where the oil was heated to 72 hours and 168 hours respectively at 150°C (Fig. 5 and Fig. 6).
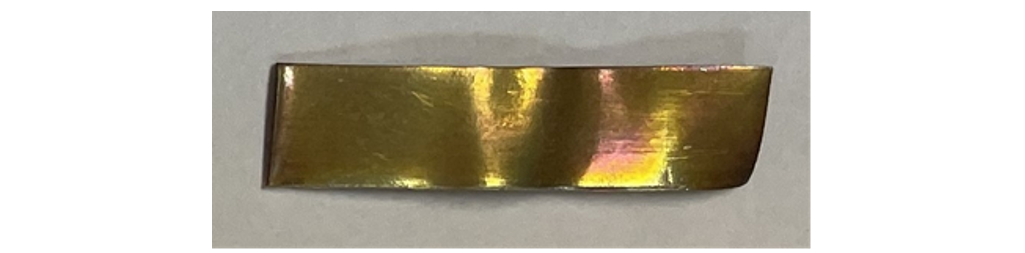
Figure 5 - Silver strip after modified ASTM D1275-15 corrosion test (72 hours at 150°C) (Energy dispersive X-ray spectroscopy (EDX) = 0.91 – 1.24 wt. % sulphur)
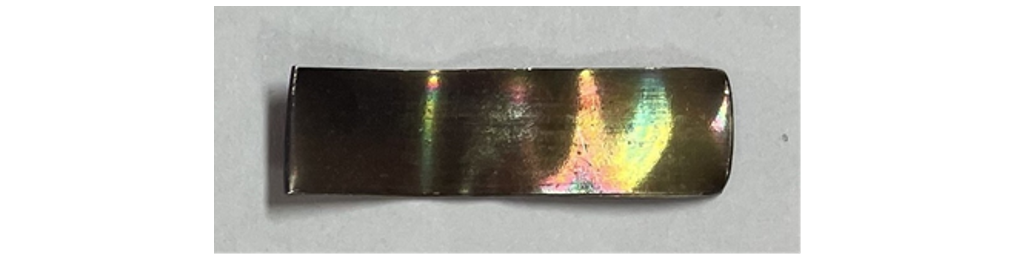
Figure 6 - Silver strip after modified ASTM D1275-15 corrosion test (168 hours at 150°C) EDX = 1.45 – 1.90 wt. % sulphur)
Cases in synthetic ester
One TF member reported that they had tested six synthetic ester-filled transformers manufactured over the last six years; all of the ester was received from the same supplier. Two 132 kV 60 MVA transformers manufactured in 2019 tested positive for corrosion to silver. A further two 66 kV 25 MVA transformers manufactured in 2023 were analysed ahead of commissioning and also found to be corrosive to silver, the ester has since been replaced with non-corrosive ester, while a similar transformer manufactured in 2021 was found not to be corrosive. An additional transformer manufactured in 2019 is still to be tested.
Ten ester-filled transformers at lower voltages, 20 kV and 30 kV, that had seen between one and fifteen years in service were inspected by a member of the TF and it was reported that nine of them had blackened coating in the de-energized tap-changers (DETC) (Fig. 7). The ester from each transformer was tested according to ASTM D1275 and, although several strips were slightly discoloured, two samples were positive for corrosion according to the Scanning Electron Microscopy (SEM)-EDX.

Figure 7 - Identified Cases, Left: Hermetic Transformer, 4.6 MVA, 20 kV, Year of Manufacture: 2020 right: Hermetic Transformer, 5 MVA, 22 kV, Year of Manufacture: 2021
An investigation was conducted by one TF member into a hermetically sealed transformer (20 kV/4.6 MVA), that that had been in operation for 2 years. The DETC contacts were evidently affected by corrosion (Fig.8) while none of the tests on the ester (ASTM D1275, DIN 51353, IEC 62535, IEC 62697-1 & 2) gave corrosive results. This finding may indicate that the corrosive sulphur compounds had reacted to the point of depletion to an undetectable level within the service life and that the sample taken during the investigation was no longer corrosive.

Figure 8 - Silver sulphide observed on DETC contacts of a 20 kV transformer
Another TF member reported a case of a 75 MVA transformer that had been in service for around 7 years. Black deposits were observed on all silver coated surfaces (Fig. 9). Some deposits had dislodged from the silver surface. No other metal surfaces were found to be affected.

Figure 9 - Blackened contacts coated with silver sulphide
Upon investigation using SEM-EDX, the deposits were confirmed to be silver sulphide with no source of sulphur identified. The synthetic ester was found to be non-corrosive according to DIN 51353. Following the test method according to ASTM D1275, the wt. % of sulphur on the silver strip was found to be 1.3%. Additionally, quantification of elemental sulphur was done and 0.31 mg/kg of S8 was measured using IEC 62697-Part 3. In another case, 13.5% wt. of sulphur was measured (using SEM-EDX) on the OLTC contact which was directly exposed-in contact with oil.
Laboratory testing
Silver corrosion tests and quantification methods should cover different types of insulating liquids and its applicability and sensitivity should be adopted for each specific liquid [17].
Many of the TF members conducted their own investigations into the corrosivity of both mineral oils and ester liquids to silver and shared their findings. As S8 is known to be particularly corrosive to silver, investigations into different quantitative methods for S8 with low limits of detection were also conducted and shared. A summary of the findings along with some of the test results are shared below, these mostly focus on testing in synthetic ester which mostly contained S8 in concentrations below 1 mg/kg and were found to be corrosive according to ASTM D1275.
Corrosive sulphur tests
Different standard test methods have been published to assess the corrosive effect of sulphur in mineral oil to both copper and silver. The table below explains the basic difference in the standard test methods available for assessing corrosion.
| Test | Material (size in mm) | Volume of sample (mL) | Time (h) | Temp (°C) | Flush with nitrogen | Vessel | Interpretation by visual inspection |
|---|---|---|---|---|---|---|---|
DIN 51353 | Silver Strip | 100 | 18 | 100 | No | Flask | Corrosive/ |
IEC 62535 [18] | Copper (7.5x1.5) wrapped in Kraft Paper | 15 | 72 | 150 | No | Crimped vial | Corrosive/ |
ASTM D1275 [5, 6] | Copper and Silver Strips | 220 | 48 | 150 | Yes | Sealed bottle | Cu – ASTM D130 [19] |
Note: The 1985 version of DIN 51353 was used most commonly by TF members. The 2021 revision is given as it is the latest version. Both versions of ASTM D1275 (2015 and 2024) were used by TF members, they differ only in interpretation of the SEM-EDX analysis.
Since the focus of the TF was silver corrosion, DIN 51353 and ASTM D1275 were the main tests carried out, although some paper wrapped copper corrosion testing using IEC 62535 was also carried out for comparison purposes.
ASTM D1275 was performed with and without nitrogen bubbling in different liquids, although it is required by the test. With synthetic oils in particular which are containing S8, elimination of oxygen through the use of nitrogen bubbling prior to sealing the liquid in a sample bottle enhances the method sensitivity and gives less ambiguity with positive results. Purging the samples beforehand with 0.5 L/min nitrogen for 5 to 10 minutes is sufficient to improve repeatability and reduce ambiguity in interpretation of the results. The presence of oxygen can significantly reduce repeatability and give false non-corrosive result. In oils which contain DBDS (almost exclusively the case with mineral oils) oxygen increases silver sulphide deposition [20]. The proposed JWG should focus on providing recommendations on the specific details for revising existing methods.
In many cases results were confirmed using SEM-EDX in addition to observation of the discoloration. SEM-EDX is recommended by ASTM in the cases of ambiguity. The TF recommendation is to run SEM-EDX analyses as an integral part of the method. ASTM D1275-24 has a higher threshold of sulphur concentration for a corrosive result (2.5%), but a lower threshold (1%), should be considered since the evidence of samples tested typically showed the range of sulphur contents from 1-1.5 wt. % and above.

Figure 10 - One test setup using in accordance with ASTM D1275
One laboratory used ASTM D1275-15 (Fig. 10) to test seven synthetic esters from five different suppliers, with several batches of one ester tested multiple times and a mineral oil (known to be non-corrosive) tested for comparison. The table below shows the total number of samples evaluated:
| Oil Type | Year of Production | Reference |
Mineral Oil 1 | 2022 | MO1-22 |
Synthetic Ester 1 | 2013 | SE1-13 |
2021 | SE1-21 | |
2022 | SE1-22 | |
2023 | SE1-23 | |
Synthetic Ester 2 | 2021 | SE2-21 |
Synthetic Ester 3 | 2016 | SE3-16 |
Synthetic Ester 4 | 2023 | SE4-23 |
Synthetic Ester 5 | 2011 | SE5-11 |
Synthetic Ester 6 | 2022 | SE6-22 |
Synthetic Ester 7 | 2017 | SE7-17 |
The results were evaluated visually in all cases and only using SEM-EDX for borderline results. Of all samples tested only SE1 gave a corrosive result for samples from 2013, 2021 and 2022, with the 2023 sample giving a non-corrosive result.
A second TF member also evaluated samples from different suppliers. In this case three were evaluated, one each from suppliers in Americas, Asia and Europe. As well as testing in their own laboratory, samples were submitted to two other independent laboratories.
IEC 62535 using wrapped copper for comparison gave negative results as did DIN 51353 for silver. However, with ASTM D1275 using silver strips, one of the esters gave a corrosive result. The two independent laboratories gave differing results with one also testing as corrosive but the other suggesting it was non-corrosive.

Figure 11 - Testing of a synthetic ester for corrosion - from left to right: DIN 51353 (non-corrosive), ASTM D1275 copper (non-corrosive), ASTM D1275 silver (corrosive), IEC 62535 copper and paper (non-corrosive)
The sensitivity of DIN 51353 and ASTM D1275 were evaluated with one mineral oil and two synthetic esters with less than 1 mg/kg of elemental sulphur. The ASTM tests were performed in specially designed ASTM bottles (Fig. 12) with polytetra-fluoroethylene (PTFE) threaded plug and a fluoro-elastomer ‘O’-ring to prevent oxygen ingress during the test.

Figure 12 - ASTM D1275 sample bottle
Use of bottles other than prescribed ASTM bottles (Fig. 12) can cause scattering of results between laboratories. If oxygen is present, even in small quantities, such as ingress through an imperfect seal on the lid of the vessel, it can lead to an absence of silver sulphide formation (i.e. a non-corrosive result), where it would otherwise have been found if the prescribed bottles were used.
Results of mineral oil testing with low concentration of elemental sulphur, using DIN 51353 and ASTM D1275-15 are obviously different, with more severe silver sulphide deposition after ASTM D 1275-15 (Fig. 13).
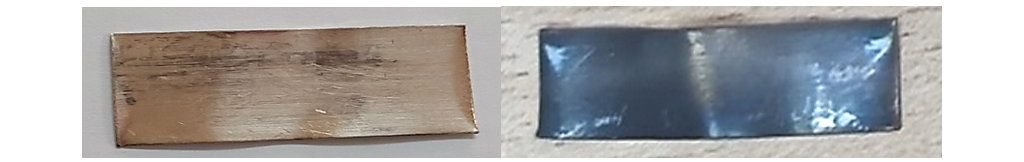
Figure 13 - Results of silver corrosion tests with minute concentrations of elemental sulphur in mineral oil (S8=0.35 mg/kg), left – DIN 51353, right ASTM D 1275-15 (EDX: S wt. %=1.54)
Synthetic esters remained non-corrosive after DIN 51353 test (Fig. 14, left). It was observed that exclusion of oxygen is very important to obtain silver sulphide deposition in the reaction of S8 with silver, as shown after ASTM D1275-15 test (without oxygen) which resulted in a substantial silver sulphide deposition (Fig. 14, right).
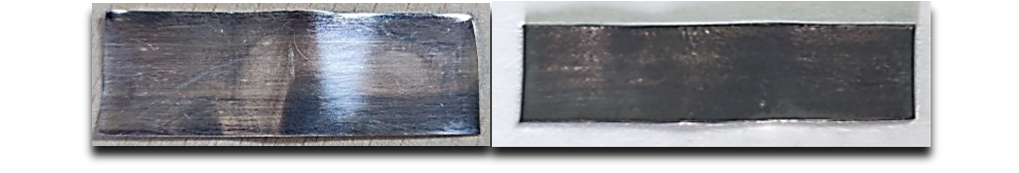
Figure 14 - Results of silver corrosion tests with minute concentrations of elemental sulphur in synthetic ester (S8=0.56 mg/kg), left – DIN 51353 (EDX: S wt. %=0.27), right - ASTM D1275-15 (EDX: S wt. %=2.30)
DIN 51353 and ASTM D1275-15 very often give different results for the same oil sample and sometimes both tests give non-corrosive result even if reactive sulphur species are present in the oil. This is due to testing conditions not adequately targeting the reactivity of sulphur species in the oil (S8 and DBDS). The DIN 51353 test was found to be applicable only for S8 in mineral oils to certain concentration range (approx. 0.5 mg/kg), while ASTM D 1275-15 was found to have good response to S8 in both mineral oils and esters. In the case of inhibited mineral oils with DBDS in the range from 40 to 200 mg/kg, in many cases ASTM D 1275-15 gave a non-corrosive result [20].
One of the TF members reported synthetic ester liquid (virgin, as delivered) tested for silver corrosion and found to contain 0.54 mg/kg of S8 with strong silver sulphide deposition, after being tested according to ASTM D1275 (Fig. 15 and Fig. 16). The liquid was manufactured in April 2024. The manufacturer advised that any subsequent batches of this liquid, manufactured after May 2024, were non-corrosive to silver.

Figure 15 - Silver corrosion observed in synthetic ester as delivered in April 2024
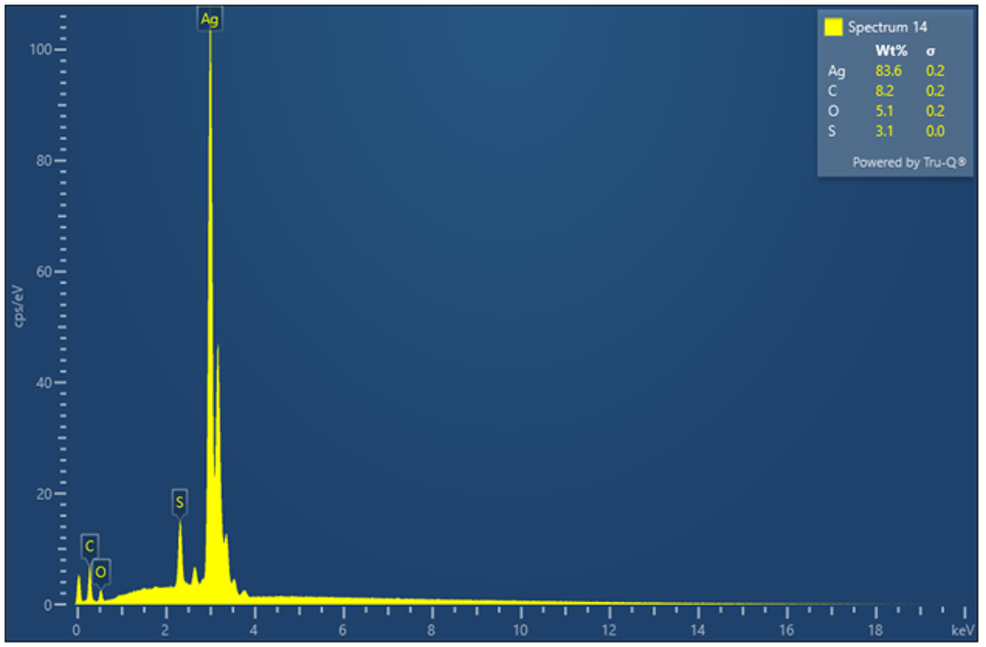
Figure 16 - Example of an EDX analysis of the silver plate (3.1 % wt. Sulphur) after ASTM D1275 testing on synthetic ester with 0.53 mg/kg of elemental sulphur
Another TF member also reported on testing of synthetic ester samples that had been taken from 13 different transformers, with all the ester insulating liquid received from the same supplier. The transformers covered three voltage levels - 400 kV, 275 kV and 33 kV - and three different manufacturers, they had been in service for between one and six years. The liquid in the main tank had been found corrosive according to DIN 51353 in three cases (one at 400 kV and two at 275 kV) and in those three cases the separate oil compartments for the OLTCs were also found to be corrosive to the same test. Since ASTM D1275 testing was not performed, it is possible that more samples would have been found corrosive as this is more sensitive. No internal inspections have been carried out yet, but these are planned to check silver contacts in the OLTCs.
It was noticed that oils which did not cause silver corrosion as delivered may become corrosive after contact with some rubber materials, such as NBR. Some materials cause severe corrosiveness even with a low ratio compared to oil. Corrosiveness of liquids after contact with different materials should be tested as part of material compatibility tests [14, 15].
Quantitative analysis of sulphur compounds corrosive to silver
Silver strip methods give an indication of the presence or absence of corrosive sulphur and the ambiguity in the execution and the evaluation can be avoided with EDX. Whilst this helps a transformer owner understand if there is a risk to the silver surfaces, additional information may be gained from a more quantified approach. Determination of the concentration of the corrosive sulphur compounds helps in at least two ways – (i) calculation of how much sulphur may have reacted if the starting and current concentrations are known and (ii) tracking the reduction of the concentration to an acceptable level if a removal technique is available.
Total sulphur may be measured using UV fluorescence [21] or atomic emission spectroscopy [22, 23] or X-ray fluorescence spectroscopy [24, 25]. However, total sulphur is probably of limited use when concerned with corrosivity, and potentially misleading. Most standard techniques are also likely to be insufficiently sensitive.
A method for measuring the concentration of DBDS in mineral oil is already published (IEC 62697 – Part 1) [7] and this TF considers that the method is also applicable to synthetic esters if there is a need.
Elemental sulphur test results
When it comes to S8, there is another part to IEC TR 62697: part 3 [8] which is applicable for quantification in synthetic esters requiring low limits of detection (LoD) and quantification (LoQ).
Polarography [26] and ultra-high performance supercritical fluid mass spectrometry (UHPSFC-MS) [27] were also used. Polarography is a very sensitive method for the detection of S8. It is a voltametric method, which makes use of a liquid mercury electrode. The method is applicable for different analytes and has short measuring times with a detection limit of 0.01 mg/kg for sulphur.
The method can be applied by using a calibration curve or by using standard additions. The method is applicable in mineral oil, and synthetic and natural ester.
The alternate method used with UHPSFC-MS involved derivatisation of the sulphur molecule with triphenyl phosphine (TPP) to produce triphenyl phosphine sulphide (TPPS). The method was originally developed to quantify S8 in mineral oil and has been found to be suitable for synthetic esters with a sensitivity close to 0.01 mg/kg.
TF members have reported on the development of S8 measurement techniques to understand the LoD and LoQ but there have not been a significant number of test results reported.
The TF member, mentioned above that had tested 13 transformers for silver corrosion according to DIN 51353, found elemental sulphur concentrations in synthetic ester, ranging from undetectable to 0.80 mg/kg across 34 samples from main tank and OLTC compartment samples, all taken from in-service transformers. These values were obtained using the UHPSFC-MS and derivatisation method referred to above. The correlation between the DIN test results and S8 measurements was poor, there was no indication that a silver strip will show as “corrosive” above an S8 threshold, which was expected.
Conclusion
This TF, that was established to report to SC A2 within 12 months for an assessment of the problems facing the industry associated with silver corrosion, has identified a need for further investigation and potential revision of current practices. The available standards for assessing silver strip corrosion, as well as the quantification methods for corrosive sulphur species, S8 in particular, were found to be in need of revision to mirror service experiences, considering both synthetic ester liquids and mineral oils. The existing standards were originally developed for a comparably higher sulphur content in mineral oils; existing methods were found to have a lack of sensitivity for different corrosive sulphur species that are found both in mineral oils and ester based insulating liquids. A new JWG should propose revision of the existing methods and/or development of new silver corrosion tests to better reflect reactivity of corrosive sulphur species and conditions in power transformers. Quantification methods should improve in reaching lower detection limits in the case of S8.
Risk assessment of power transformers should be based on the propensity of insulating liquids to react with silver. Besides the type and concentration of reactive sulphur compounds in insulating liquid, the intensity of the reaction depends on number of factors such as: total surface area of silver and its ratio to liquid, load conditions in correlation to the temperature profile. The consequences of the presence of corrosive sulphur should consider the extent of corrosion on the surface starting from light discolorations of silver surfaces which may not be harmful for reliable transformer operation, to heavy corrosion that involves silver whiskers or flaking of silver sulphide particles which can be critical for transformer operation leading to its failure.
Kinetic studies showing low activation energy of the reaction between elemental sulphur and silver, including high oil to silver surface ratio as presented in recent papers [28, 29] can provide the explanation of silver sulphide deposition from insulating liquids containing S8 in sub-ppm concentration range at low operating temperatures, as observed in the field. The mechanism and kinetics of the reaction of sulphur species with silver in different insulating liquids, as well as failure modes and compatibility affecting corrosion (how can sulphur dissolved in the oil from other materials cause corrosion effects?) should be further investigated. Guidelines for risk assessment, condition monitoring and inspection methods, remedial actions as well as short-term and long-term mitigation techniques should be recommended (change and treatment of liquids, change and cleaning of corroded contacts), considering materials compatibility to provide sustainable solutions. While the focus of the TF has been on corrosivity to silver in synthetic esters and mineral oil, the JWG should give some regard to the appropriateness of currently available guidance for copper corrosion, and other liquids, such as natural esters, should not be ignored.
References
- TB 378 "Copper sulphide in transformer insulation" Dahlund, M; et al., CIGRE, Paris, France, 2009.
- TB 625 "Copper Sulphide Long Term Mitigation and Risk Assessment" Lukic, J; et al., CIGRE, Paris, France, 2015.
- DIN 51353 Testing of insulating oils; detection of corrosive sulfur; silver strip test, Berlin, Germany: Deutsches Institut für Normung, 1985.
- DIN 51353 Testing of insulating oils - Detection of corrosive sulfur - Silver strip test, Berlin, Germany: Deutsches Institut für Normung, 2021.
- ASTM D1275-15 Standard Test Method for Corrosive Sulfur in Electrical Insulating Liquids, West Conshohocken, PA: ASTM International.
- ASTM 1275-24 Standard Test Method for Corrosive Sulfur in Electrical Insulating Liquids, West Conshohocken, PA: ASTM International.
- IEC 62697-1:2012 Test methods for quantitative determination of corrosive sulfur compounds in unused and used insulating liquids - Part 1: Test method for quantitative determination of dibenzyldisulfide (DBDS), Geneva, Switzerland: International Electrotechnical Commission.
- IEC TR 62697-3:2018 Test methods for quantitative determination of corrosive sulfur compounds in unused and used insulating liquids - Part 3: Test method for quantitative determination of elemental sulfur, Geneva, Switzerland: International Electrotechnical Commission.
- M. Dahlund, H. Johansson, U. Lager and G. Wilson, “Understanding the Presence of Corrosive Sulfur in Previously Non-Corrosive Oils Following Regeneration,” in International Conference of Doble Clients, Boston, MA, 2010.
- J. Lukic, J. Jankovic, Planojevic, M. Foata, Zieglschmid, Castano and Briotto, “Silver Sulphide in OLTCs—Root Causes and Proactive Mitigation Strategies,” in Asia-Pacific TechCon, Sydney, Australia, 2019.
- A. F. Holt, M. Facciotti, P. S. Amaro, R. C. D. Brown, P. Lewin, J. A. Pilgrim, G. Wilson and P. Jarman, “An Initial Study Into Silver Corrosion in Transformers Following Oil Reclamation,” in IEEE Electrical Insulation Conference (EIC), Ottawa, ON, 2013.
- F. Scatiggio, M. Pompili and R. Bartnikas, “Oils with Presence of Corrosive Sulphur: Mitigation and Collateral Effects,” in IEEE Electrical Insulation Conference (EIC), Montral, QC, 2009.
- V. Tumiatti, R. Maina, F. Scatiggio, M. Pompili and R. Bartnikas, “Corrosive sulphur in Mineral Oils: Its detection And Correlated Transformers Failures,” in IEEE International Symposium on Electrical Insulation (ISEI), Vancouver, BC, 2006.
- V. Haramija, B. Musulin, D. Vrsaljko and V. Ðurina, “Consequences of rubber incompatibility with transformer oil,” in 5th International Colloquium Transformer Research and Asset Management, Split, Croatia, 2019.
- D. Vrsaljko, V. Ðurina, V. Haramija and T. Cvrtila, “Chemistry in transformers: paper and rubber,” in CIGRE SC A2 and 6th International Colloquium Transformer Research and Asset Management Joint Colloqiuium, Split, Croatia, 2023.
- L. R. Lewand, “The Role of Corrosive Sulfur in Transformers and Transformer Oil,” 2002. [Online].
- IEC 61039:2025 Classification of insulating liquids, Geneva, Switzerland: International Electrotechnical Commission.
- IEC 62535:2008 Insulating liquids - Test method for detection of potentially corrosive sulphur in used and unused insulating oil, Zurich, Switzerland: International Electrotechnical Commission.
- ASTM D130-19 Standard Test Method for Corrosiveness to Copper from Petroleum Products by Copper Strip Test, West Conshohocken, PA: ASTM International.
- J. Jankovic, J. Lukic, D. Mihajlovic, M. Foata and B. Myburgh, “Development of a New Method for the Assessment of Mineral Insulating Oil Corrosivity Against Silver,” IEEE Transactions on Dielectrics and Electrical Insulation, vol. 31, no. 4, pp. 2169-2178, 2024.
- ASTM D5453-09 Standard Test Method for Determination of Total Sulfur in Light Hydrocarbons, Spark Ignition Engine Fuel, Diesel Engine Fuel, and Engine Oil by Ultraviolet Fluorescence, West Conshohocken, PA: ASTM International.
- ASTM D5185-09 Standard Test Method for Determination of Additive Elements, Wear Metals, and Contaminants in Used Lubricating Oils and Determination of Selected Elements in Base Oils by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES), West Conshohocken, PA: ASTM International.
- ASTM D7151-15 Standard Test Method for Determination of Elements in Insulating Oils by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES), West Conshohocken, PA: ASTM International.
- ASTM D2622-21 Standard Test Method for Sulfur in Petroleum Products by Wavelength Dispersive X-ray Fluorescence Spectrometry, West Conshohocken, PA: ASTM International.
- ASTM D4294-24 Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry, West Conshohocken, PA: ASTM International.
- Metrohm, Application Note No. V-85 "Elemental sulfur in gasoline".
- S. B. Garcia, J. Herniman, P. Birkin, J. Pilgrim, P. Lewin, G. Wilson, G. J. Langley and R. C. D. Brown, “Quantitative UHPSFC-MS analysis of elemental sulfur in mineral oil via derivatisation with triphenylphosphine: application to corrosive sulfur-related power transformer failure,” Analyst, vol. 145, pp. 4782-4786, 2020.
- J. Lukic, J. Jankovic, D. Mihajlovic, S. Glisic and A. Orlovic, “Silver Corrosion Testing and Mitigation,” Ref. D1-10893-2024 in CIGRE Session, Paris, 2024.
- J. Lukic, J. Jankovic, V. Vasovic and D. Kolarski, “Silver Corrosion Risk Assessment and Mitigation,” in 92nd International Conference of Doble Clients, Boston, MA, 2025.
